Our Services
Our Services
MiNDNET develops evidence-based digital health applications for people with mental illness. Our digital therapeutics meet the highest standards of innovative development, scientifically proven efficacy, user-friendliness, application flexibility and data security integrated into the existing healthcare system and support doctors and therapists in the treatment process.
We ensure the approval
AS an ISO 13485 and ISO 27001 certified company, we take care of the entire approval process. We ensure the certification of the solution as medical device and act on request as official manufacturer. We coordinate the admission studies, and take over the registration with the BfArM or other local institutions.
We orchestrate stakeholders
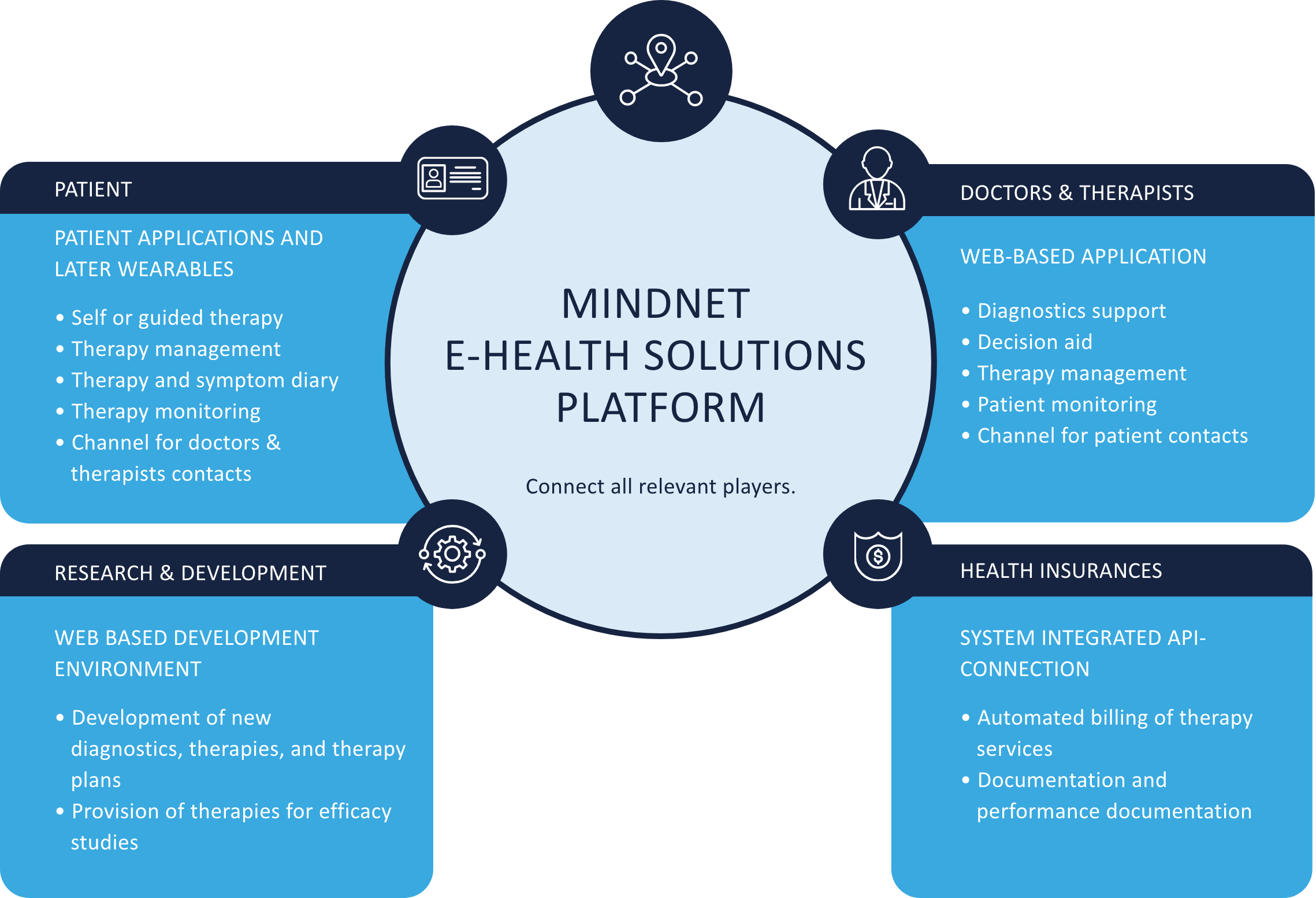
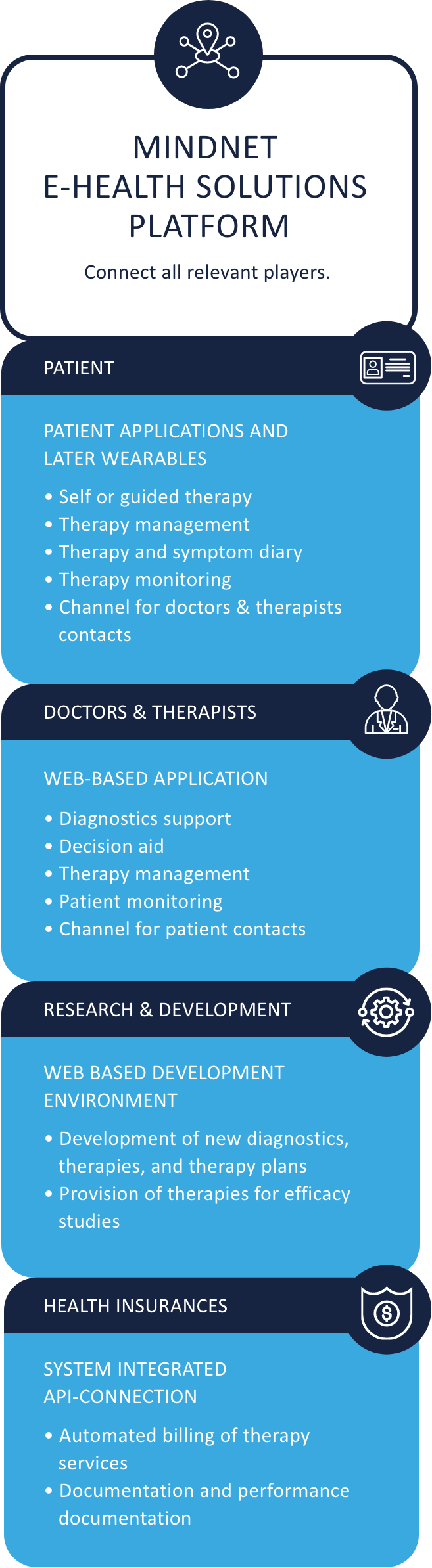
Several stakeholders are involved in developing digital mental health therapeutics, including patients, health care practitioners, researchers, traditional medical device industry firms, mobile application developers, and the designated body and BfArM for the medical device and DiGA approval. MiNDNET connects all stakeholders and takes care of relevant processes and communication. Permitting successful implementation.
MinDNET digital platform strategy

From Idea to Operation
MiNDNET supports the entire development process of a digital therapeutic. This includes consulting, conception, production of clickable prototypes, minimal viable products and market-ready digital therapeutics, validation through qualitative and randomized controlled trials, market approval and operation.
Consulting
We advise you on all key aspects of digital health applications including health policy situation, evidence-based development, market and competition analysis, possible business models, and market introduction.
Research
We support the entire conception of the digital therapeutic including evidence-based fundamentals regarding guideline-based treatment and digital therapy, creation of a therapy concept, personas and customer Journeys.
Clickable prototype
Clickable prototypes give an initial impression of the functions and content of the digital therapeutic. Customers can thus experience and precisely understand the functions and content of the digital health application. It can be used to obtain feedback from experts and sufferers and to conduct market research.
Minimal viable product
A Minimal Viable Product (MVP) is a product with enough features to validate the product early in the product development cycle. MVP can help the product team get user feedback as quickly as possible to iterate and improve the product_ Together with our partner universities, we also conduct qualitative pilot studies with patients and professionals. Here, above all, the applicability is tested and used directly to improve the MVP.
Market-ready digital therapeutics
Market-ready digital therapeutics have gone through the development steps and meet all specifications regarding data protection and quality assurance. They can be provisionally approved. Regulatory studies can be conducted with them.
Validation
With our university partners, we conduct randomized controlled trials on the efficacy and safety of the digital therapeutic. Here, we have over 25 years of experience and have conducted over 50 large RCTs within our university background. Our two partner universities in Hamburg and Bern have over 300 study publications on digital therapeutics.
Approval
We support the approval process of the digital therapeutic or carry it out ourselves. This also includes preparing all documents or conducting price negotiations with the regulatory authority.
Support
Physicians prescribe and patients use our digital therapeutics, while we gain and apply real-world Insights to continuously Improve the applications.
Interested in partnering with us?
→ Let us shape the future of e-health solutions together!